to assess the epidemiological and genetic factors associated with severity of acute viral bronchiolitis (AVB) by respiratory syncytial virus (RSV).
Data sourcethe key words “bronchiolitis”, “risk factor”, “genetics” and “respiratory syncytial virus”, and all combinations among them were used to perform a search in the PubMed, SciELO, and Lilacs databases, of articles published after the year 2000 that included individuals younger than 2 years of age.
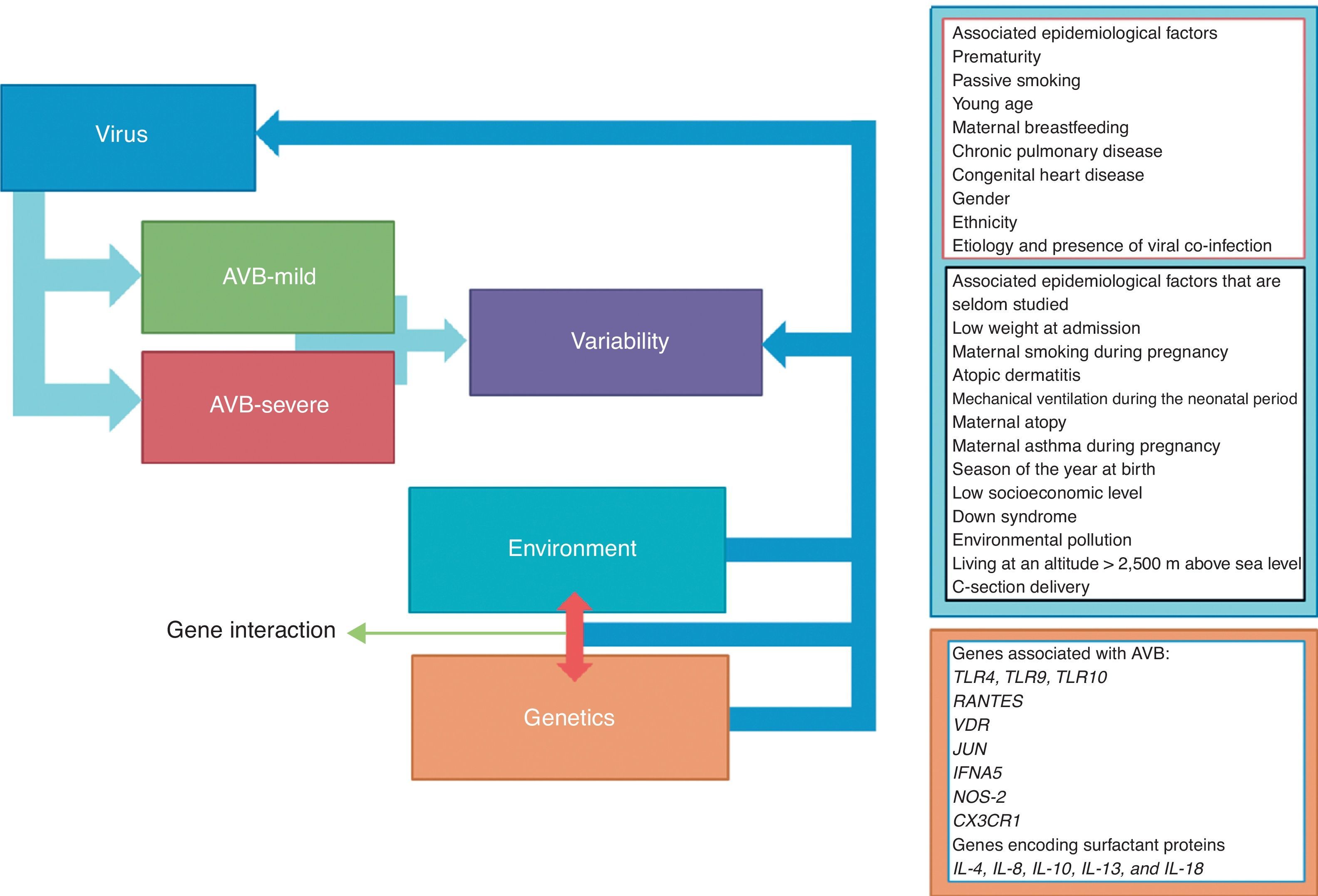
Data synthesisa total of 1,259 articles were found, and their respective summaries were read. Of these, 81 were selected, which assessed risk factors for the severity of AVB, and were read in full; the 60 most relevant studies were included. The epidemiologic factors associated with AVB severity by RSV were prematurity, passive smoking, young age, lack of breastfeeding, chronic lung disease, congenital heart disease, male gender, ethnicity, viral coinfection, low weight at admission, maternal smoking during pregnancy, atopic dermatitis, mechanical ventilation in the neonatal period, maternal history of atopy and/or asthma during pregnancy, season of birth, low socioeconomic status, Down syndrome, environmental pollution, living at an altitude > 2,500 meters above sea level, and cesarean section birth. Conversely, some children with severe AVB did not present any of these risk factors. In this regard, recent studies have verified the influence of genetic factors on the severity of AVB by RSV. Polymorphisms of the TLRs, RANTES, JUN, IFNA5, NOS2, CX3CR1, ILs, and VDR genes have been shown to be associated with more severe evolution of AVB by RSV.
Conclusionthe severity of AVB by RSV is a phenomenon that depends on the varying degrees of interaction among epidemiological, environmental, and genetic variables.
avaliar os fatores epidemiológicos e genéticos associados à gravidade da Bronquiolite Viral Aguda (BVA) pelo Vírus Sincicial Respiratório (VSR).
Fonte dos dadosforam utilizados descritores “bronchiolitis”, “risk factor”, “genetics” e “respiratory syncytial virus” e todas as combinações entre eles, nas bases de dados PubMed, SciELO e Lilacs publicados após o ano de 2000 e que incluíram indivíduos menores de dois anos de idade.
Síntese dos dadosforam encontrados 1.259 artigos e lidos seus respectivos resumos. Destes foram selecionados 81 que avaliaram fatores de risco para a gravidade da BVA para leitura na íntegra, e foram incluídos os 60 estudos mais relevantes. Os fatores epidemiológicos associados com a gravidade da BVA pelo VSR foram: prematuridade, tabagismo passivo, baixa idade, ausência de aleitamento materno, doença pulmonar crônica, cardiopatia congênita, sexo masculino, etnia, coinfecção viral, baixo peso na admissão hospitalar, tabagismo materno na gestação, dermatite atópica, ventilação mecânica no período neonatal, antecedente materno de atopia e/ou asma na gestação, estação do nascimento, baixo nível socioeconômico, síndrome de Down, poluição ambiental, morar em altitude acima de 2.500 metros do nível do mar e parto cesariana. Em contrapartida, algumas crianças com BVA grave não apresentam nenhum desses fatores de risco. Neste sentido, estudos recentes têm verificado a influência de fatores genéticos relacionados à gravidade da BVA pelo VSR. Polimorfismos dos genes TLRs, RANTES, JUN, IFNA5, NOS2, CX3CR1, ILs e VDR têm-se mostrado associados com a evolução mais grave da BVA pelo VSR.
Conclusãoa gravidade da BVA pelo VSR é um fenômeno dependente da interação entre variáveis epidemiológicas, ambientais e genéticas em seus diferentes graus de interação.
Acute viral bronchiolitis (AVB) caused by respiratory syncytial virus (RSV) is the primary infection of the lower airways in children under 2 years of age worldwide, and it is the main cause of hospitalization in this age group in developed countries.1 Although all children are infected with RSV by the age of three, most infections are mild and have no sequelae. The mechanisms involved with the severity of AVB caused by RSV are not yet fully understood. Why does RSV infection present such variable evolution in different patients? Why does one child with RSV remain asymptomatic and another child dies? When assessing the severity of AVB caused by RSV, which factors are more often associated: genetic and/or epidemiological/environmental factors? These questions have intrigued researchers and remain without definitive answers.
There are 3,000 to 4,000 deaths annually in the United States due to AVB caused by RSV.2 The prevalence of hospitalization due to RSV in the United States is 48.9 per 1,000 in children younger than 3 months, 26 per 1,000 in those younger than 1 year, and 1.8 per 1,000 in children aged 1 to 5 years, with 132,000 to 172,000 hospitalizations/year due to RSV in children under 5 years.3 In the United States, there are, on average, 22.8 visits to the emergency room caused by RSV per 1,000 infants; 29% of whom are hospitalized. That represents an annual spending of 50.5 million dollars on emergency room visits and 650 million dollars on hospitalizations.4 In other regions, the rate of hospitalization per 1,000 infants with RSV varies from 8.7 in Australia5 to 60 in Japan.6 In Australia, the incidence of RSV is from 110.0 to 226.5 per 1,000 infants, and the annual cost is estimated at $ 50 million dollars, which is more significant than the costs of Influenza and Rotavirus infections.5 In Europe, RSV is responsible for 45% of hospitalizations for lower respiratory infection in children younger than 2 years.6
In Brazil, a study of 5,304 children younger than 1 year showed that 113 (2.1%) were hospitalized due to AVB.7 Among the children hospitalized for RSV, 2.7% were admitted to the intensive care unit (ICU), 1.5% required assisted ventilation, and 0.2% died.8
Infection by RSV has variable severity with clinical manifestations from mild symptoms in the upper respiratory tract to bronchiolitis and pneumonia, and may develop into the severe form, requiring ICU admission and mechanical ventilation, and at times leading to death. To date, the treatment of AVB by RSV is supportive. It has been demonstrated that, in the United States, of 1.1 million children younger than two years hospitalized for RSV in a period of eight years, the highest percentage of hospitalizations occurred between 3 and 6 months of age, thus affecting a population range at risk of mortality.9 The first infections are usually symptomatic and often affect the lower airways; subsequent infections are usually milder.
RSV is the agent responsible for AVB in 41.7%10 to 83.6% of cases.11 In Brazil, RSV was responsible for 31.9%12 to 64% of hospitalized patients with AVB.13 Although other viruses are detected in patients with AVB, such as adenovirus, bocavirus, Influenza A, Influenza B, Parainfluenza virus 1, Parainfluenza virus 2, Parainfluenza virus 3, rhinovirus, and metapneumovirus,10,11 the establishment of codetection or coinfection has been a critical aspect yet to be considered.14 The second most common virus in AVB is the rhinovirus, corresponding to approximately 18% of cases.15 In Brazil, it was found that viral coinfection occurs in 40% of AVB cases and that the most common virus after RSV is the rhinovirus, occurring in 40% of cases.16
Epidemiological factors associated with the severity of AVB by RSV are known and have been reported in the literature; however, some children with severe AVB do not have any of these risk factors (Table 1). In this regard, recent studies have assessed the influence of genetic factors related to disease severity (Table 2).
Studies of epidemiological variables associated with Acute Viral Bronchiolitis related to Respiratory Syncytial Virus included in the literature review.
| Variable | Author | Year | n | Main findings |
|---|---|---|---|---|
| Prematurity | Gouyon et al.18 | 2012 | 498 infants younger than 6 months hospitalized with AVB | Seven-fold higher risk of AVB by RSV |
| Nascimento et al.16 | 2010 | 77 patients with AVB | Higher probability of ICU admission – OR: 24.51 (95% CI: 3.21–186.92) | |
| Grimwood et al.22 | 2008 | 230 infants | Higher probability of hospitalization | |
| López Guinea et al.23 | 2007 | 284 patients admitted at the ICU for AVB | 30% were premature | |
| Albernaz et al.7 | 2003 | 5.301 children followed for one year -113 hospitalized for AVB | Risk of hospitalization 80% higher in children of mothers whose pregnancies lasted less than 37 weeks | |
| Chan et al.24 | 2002 | 216 infants hospitalized with AVB by RSV | Increased risk of hypoxemia and respiratory failure requiring mechanical ventilation | |
| Garcia et al.25 | 2010 | 4,800 infants hospitalized with AVB | Prematurity and positivity for RSV - risk factors for disease severity | |
| Passive smoking | Semple et al.19 | 2011 | 378 patients hospitalized with AVB | Risk factor for needing supplemental oxygen and mechanical ventilation |
| Chatzimichael et al.26 | 2007 | 240 children hospitalized with AVB | Greater clinical severity and longer hospital stay duration | |
| Jones et al.27 | 2011 | Meta-analysis of 60 studies | Risk factor for AVB | |
| Bradley et al.28 | 2005 | 206 patients hospitalized with AVB by RSV | Children exposed to postnatal maternal smoking had lower levels of hemoglobin oxygen saturation than the non-exposed ones (89.8% versus 92.2%, p = 0.01) | |
| Albernaz et al.7 | 2003 | 5,301 children followed for one year -113 hospitalized for AVB | Risk of hospitalization for AVB - 57% higher in children exposed to maternal smoking than in unexposed ones | |
| Young age | Nascimento et al.16 | 2010 | 77 infants treated at the ER for AVB | OR = 0.838 (95% CI: 0.718–0.979) related to hospitalization - older age associated with lower incidence of hospitalization |
| Hervás et al.29 | 2012 | 2384 infants hospitalized with AVB | Patients younger than two months had longer hospital stay duration (6 versus 5 days, p<0.00001) and increased risk of ICU admission (OR = 3.4; 95%CI: 2.5-4,6) | |
| Oñoro et al.30 | 2011 | 229 patients admitted at ICU with AVB | Age is inversely proportional to the time needed for ICU stay and ventilatory support | |
| Damore et al.31 | 2008 | 1.456 patients with AVB | Age younger than two months - a risk factor for hospitalization in ICU (26% versus 53%; OR = 4.1; 95%CI: 2.1-8.3) | |
| Papoff et al.32 | 2011 | 310 patients younger than 12 months with AVB | Younger age = main factor associated with clinical severity | |
| López Guinea et al.23 | 2007 | 284 patients admitted at ICU for AVB | Age younger than six weeks = main risk factor, accounting for 45% of patients | |
| Bradley et al.28 | 2005 | 206 patients hospitalized with AVB by RSV | The younger the child, the lower are the levels of hemoglobin oxygen saturation. For each month younger in age, the child shows a reduction of 0.41% in oxygen saturation | |
| Vidaurreta et al.33 | 2011 | 347 patients with acute respiratory infection, 234 hospitalized and 112 nonhospitalized | Age of hospitalized patients was younger (8 versus 19 months, p < 0.001) | |
| Maternal breastfeeding | Koehoorn et al.20 | 2008 | 12.474 children with bronchiolitis – 1,588 hospitalized | Failure initiating breastfeeding in the maternity ward was a greater risk factor for hospitalization for AVB |
| Dornelles et al.34 | 2007 | 175 children hospitalized with AVB | Duration of exclusive breastfeeding is inversely proportional to the time of oxygen use and length of stay - for each month of exclusive breastfeeding, the time of oxygen use decreased 11 hours | |
| Albernaz et al.7 | 2003 | 5,301 children followed for one year -113 hospitalized for AVB | Children weaned before one month of age - 7.7 times greater risk of being hospitalized for AVB | |
| Chatzimichael et al.26 | 2007 | 240 children hospitalized for AVB | Maternal breastfeeding for less than four months - risk factor for severe outcome and longer hospital stay | |
| Chronic pulmonary disease | Ochoa Sangrador et al.21 | 2010 | Review study with 127 articles | Chronic lung disease, especially bronchopulmonary dysplasia - more severe AVB |
| Al-Shehri et al.35 | 2005 | 166 children younger than 5 years diagnosed with AVB, 51 hospitalized and 115 nonhospitalized | ||
| Che et al.36 | 2012 | 27,500 infants younger than 1 year hospitalized for AVB | Infants with bronchopulmonary dysplasia have 6.7 greater risk of death for AVB | |
| Congenital heart disease | Ochoa Sangrador et al.21 | 2010 | Review study with 127 articles | Greater AVB severity |
| Hervás et al.29 | 2012 | 2.384 infants hospitalized for AVB | Longer hospital stay (6 versus 5 days, p<0.0001) | |
| Fjaerli et al.37 | 2004 | 764 patients hospitalized for AVB | Presence of congenital heart disease associated with 50% longer length of hospital stay than in children without heart disease | |
| Gender | Semple et al.19 | 2011 | 378 infants hospitalized for AVB | Male gender with AVB - increased risk of needing supplemental oxygen and mechanical ventilation |
| Koehoorn et al.20 | 2008 | 12,474 children with bronchiolitis - 1.588 hospitalized | Male gender - higher risk of hospitalization | |
| Ethnicity | Bradley et al.28 | 2005 | 206 patients hospitalized with AVB by RSV | Patients of African descent have better evolution when compared to Caucasians |
| Leader et al.4 | 2003 | 330,284 infants treated at the ER for AVB | Patients of African descent have higher risk of death when compared to Caucasians | |
| Grimwood et al.22 | 2008 | 141 patients hospitalized for AVB by RSV | New Zealand - patients of Maori descent have worse evolution | |
| Meissner et al.38 | 2003 | Review study with 31 articles | Native Americans and those from Alaska have more severe disease than Caucasians38 | |
| Etiology and presence of viral coinfection | Garcia et al.11 | 2010 | 4800 infants hospitalized for AVB | Some studies suggest that RSV is a factor of AVB severity when compared to other viruses11,32 |
| Papoff et al.32 | 2011 | 310 patients younger than 12 months with AVB | Some studies suggest that RSV is a factor of AVB severity when compared to other viruses11,32 | |
| Hervás et al.29 | 2012 | 2,384 infants hospitalized for AVB | Severe AVB by RSV leads to prolonged hospitalization (6 versus 5 days, p<0,0001), higher risk of ICU admission (OR: 2.7; 95% CI: 1.87-3.9) and higher need of oxygen therapy (OR: 2.2; 95% CI: 1.8-2.6) | |
| Riccetto et al.39 | 2006 | 152 infants hospitalized for acute lower respiratory infections | Pulse oximetry < 90% at hospitalization for lower respiratory tract infection was associated with RSV infection | |
| D’Elia et al.40 | 2005 | 89 patients hospitalized | There was no difference in severity between patients with RSV and those who did not | |
| Ochoa Sangrador et al.21 | 2010 | Review study with 127 articles | Viral coinfection is responsible for more severe AVB | |
| Brand et al.42 | 2011 | 142 infants with AVB | Viral coinfection does not increase the severity of AVB | |
| De Paulis et al.43 | 2011 | 176 patients | Clinical severity of AVB by RSV does not increase due to the presence of viral coinfection | |
| Low weight at admission | Semple et al.19 | 2011 | 378 patients hospitalized wth AVB | Low weight at admission is associated with higher risk of requiring mechanical ventilation |
| Maternal smoking during pregnancy | Koehoorn et al.20 | 2008 | 12.474 children with bronchiolitis - 1.588 hospitalized | Maternal smoking during pregnancy increases risk of hospitalization for AVB |
| Carroll et al.45 | 2007 | 101,245 infants, of which 20,249 had AVB | Maternal smoking during pregnancy increases risk of AVB | |
| Atopic dermatitis | Al-Shehri et al.35 | 2005 | 166 children younger than 5 years diagnosed with AVB, 51 hospitalized and 115 nonhospitalized | History of atopic dermatitis increases risk of hospitalization for AVB |
| Mechanical ventilation in the neonatal period | Ochoa Sangrador et al.21 | 2010 | Review study with 127 articles | History of neonatal mechanical ventilation increases length of hospital stay and the possibility of ICU admission in patients with AVB |
| Maternal history of atopy | Miller et al.15 | 2011 | 630 infants with AVB | Maternal history of atopy increases AVB severity |
| Maternal history of asthma during pregnancy | Carroll et al.45 | 2007 | 101,245 infants, of which 20,249 had AVB | History of maternal asthma during pregnancy increases risk of AVB |
| Birth season | Grimwood et al.22 | 2008 | 230 infants hospitalized du to AVB | Being born between the months of February and July increases the risk of hospitalization for AVB in New Zealand |
| Low socioeconomic level | Koehoorn et al.20 | 2008 | 12,474 children with bronchiolitis – 1,588 hospitalized | Low socioeconomic level increases the risk of hospitalization for AVB |
| Down Syndrome | Fjaerli et al.37 | 2004 | 764 patients hospitalized for AVB | Down Syndrome increases AVB severity |
| Bloemers et al.46 | 2007 | 395 patients with Down Syndrome | Down Syndrome increases the risk of hospitalization for AVB | |
| Environmental pollution | Karr et al.47 | 2007 | 18,595 infants younger than 1 year hospitalized for AVB and 169,472 controls | Environmental pollution increases the risk of hospitalization for AVB |
| Living at altitude > 2,500 m above sea level | Choudhuri et al.48 | 2006 | 4,847 infants hospitalized for AVB by RSV | Living at an altitude higher than 2500 meters above sea level increases the risk of hospitalization for AVB |
| C-section delivery | Moore et al.49 | 2012 | 212,068 infants of which 7,062 were hospitalized for AVB | C-section delivery increases the risk of hospitalization for AVB |
Genes associated with severity of acute viral bronchiolitis related to respiratory syncytial virus, included in the literature review.
| Author | Year | n | Gene | Polymorphism | Main findings |
|---|---|---|---|---|---|
| Tal et al.51 | 2004 | 99 infants hospitalized for AVB by RSV and 172 controls | TLR4 | Asp299Gly (rs4986790) and Thr399Ile (rs4986791) | The presence of these polymorphisms are associated with greater severity of AVB |
| Douville et al.52 | 2010 | Evaluation of immune response in 200 patients aged 7 to 9 years | TLR4 | Asp299Gly (rs4986790) and Thr399Ile (rs4986791) | The presence of these polymorphisms does not influence the immune response |
| Löfgren et al.53 | 2010 | 312 patients with AVB by RSV and 356 controls | TLR4 | Asp299Gly (rs4986790) | There is no association between the polymorphism presence and AVB severity |
| Mandelberg et al.54 | 2006 | 52 patients with AVB by RSV | TLR4 | Asp299Gly (rs4986790) and Thr399Ile (rs4986791) | The presence of these polymorphisms is associated with greater AVB severity |
| Puthothu et al.55 | 2006 | 131 infants with AVB by RSV and 270 controls | TLR4 | D259G and T359I | The presence of a gene haplotype with 2 polymorphism is associated with increased AVB severity |
| Mailaparambil et al.56 | 2008 | TLR 1, 2, 3, 5, 6, 9 and 10 | 19 polymorphisms were evaluated | Polymorphisms in TLR 9 and 10 are associated with AVB severity | |
| Amanatidou et al.57 | 2008 | 106 children hospitalized for AVB by RSV and 120 controls | RANTES | -28C/G, -403G/A and In1.1T/C | The presence of a gene haplotype with 3 polymorphisms is associated with increased AVB severity |
| Kresfelder et al.58 | 2011 | 296 patients with AVB by RSV and 113 controls | Vitamin D receptor | Thr1Meth (rs10735810) | The T “minor” allele has greater propensity to AVB |
| Janssen et al.59 | 2007 | 470 children hospitalized for AVB by RSV and 1008 controls | 220 genes evaluated | 384 polymorphisms were evaluated | Polymorphisms in genes of the innate immune response in the Vitamin D receptor (rs10735810), JUN (rs11688), IFNA5 (rs10757212) and NOS2 (rs1060826) show strong association with AVB |
| Amanatidou et al.60 | 2006 | 82 children hospitalized for AVB by RSV and 120 controls | CX3CR1 receptor | V249I and T280M | The T280 polymorphism is associated with increased AVB severity |
| Ampuero et al.61 | 2011 | 118 infants younger than 6 months with RSV infection and 104 controls | SP-A1, SP-A2 and SP-D surfactant proteins | 11 polymorphisms were evaluated | The presence of gene haplotypes is associated with the severity of RSV infection |
| Mulet et al.62 | 2010 | Review article analyzing 16 articles | IL-4, IL-8, IL-10, IL-13 and IL-18 | Several polymorphisms were evaluated | Association between different polymorphisms and haplotypes in these genes with greater severity of RSV infection |
Due to the possibility of AVB evolving into a more severe form, it becomes important to identify genetic and environmental risk factors that can contribute to its greater severity.
Recently, several studies have led to the creation of guidelines around the world, showing that children at high risk of acquiring severe RSV infection should receive passive immunization with monoclonal antibody against RSV (palivizumab), which promotes protection against severe forms of the disease. After the introduction of palivizumab, a 48% reduction in hospitalizations of infants with chronic lung disease of prematurity was observed.17 A vaccine aimed at preventing AVB by RSV has yet to be developed, despite efforts in this regard since the 1960s.1
The objective of this review was to evaluate the epidemiological and genetic factors that contribute to the severity of AVB by RSV, allowing for better patient management and prediction of risk groups associated with the disease, decreasing costs for the health system, and allowing for a reduction in the number of hospitalizations and deaths.
MethodsThe key words “bronchiolitis”, “risk factor”, “genetics”, and “respiratory syncytial virus” and all their combinations were used in a search conducted at the PubMed (http://www.ncbi.nlm.nih.gov/pubmed), SciELO (http://www.scielo.org/php/index.php), and LILACS (http://lilacs.bvsalud.org/en/) databases of articles published after the year 2000 that included individuals younger than 2 years old. The last search was performed in October of 2012.
A total of 1,259 articles were found, of which all abstracts were read. Of these, 81 that assessed risk factors for AVB severity were selected to be read in full, and the 60 most relevant studies were included in the analysis (Fig. 1).
Results and discussionRisk factorsThe association of genetic and epidemiological/environmental factors as precursors for severe AVB by RSV has been reported in the literature. Certain risk factors are better known and are associated with disease severity, including prematurity,18 passive smoking,19 younger age,16 absence of breastfeeding,20 chronic lung disease, and congenital heart disease.21 However, other factors, such as genetic factors and other epidemiological data, show no evident confirmatory associations, and require further studies as markers of severity in AVB caused by RSV (Fig. 2).
Prematurity, without the presence of bronchopulmonary dysplasia, has a seven-fold higher risk factor of AVB by RSV.18 A Brazilian study demonstrated that in 77 patients with AVB prematurity was associated with a higher probability of ICU admission, with an OR of 24.51 (95% CI: 3.21 to 186.92).16 In 230 infants age < 24 months, prematurity (gestational age < 37 weeks) was a risk factor for hospitalization.22 Among 284 patients admitted to the ICU for AVB, 30% were preterm.23 Another Brazilian cohort study of 5,301 children followed for a year showed that 113 were hospitalized for AVB, and the risk of hospitalization was 80% higher in children whose mothers had pregnancies lasting less than 37 weeks.7 Prematurity in patients with AVB by RSV was also associated with increased risk of hypoxemia and respiratory failure requiring mechanical ventilation.24 In a retrospective analysis of 4,800 infants hospitalized for AVB, it was concluded that prematurity and positive RSV represented risk factors for disease severity.25
Passive smokingIn the assessment of 378 hospitalized patients with AVB, passive smoking was a risk factor for need for supplemental oxygen and mechanical ventilation,19 and in another sample of 240 children hospitalized for AVB, it was observed that passive smoking was associated with greater clinical severity and longer hospitalization.26 In a meta-analysis that included 60 studies, passive smoking was a significant risk factor for AVB.27 In a prospective analysis of 206 patients hospitalized for AVB by RSV, children exposed to postnatal maternal smoking had lower levels of hemoglobin oxygen saturation than those not exposed (89.8% versus 92.2%, p=0.01).28 In Brazil, it was demonstrated that the risk of hospitalization for AVB was 57% higher in children exposed to maternal smoking than those not exposed.7
Young ageIt has been well established in the literature that the younger the child, the greater the clinical severity of AVB by RSV. In a Brazilian study, age had an OR of 0.838 (95% CI: 0.718-0.979) compared to hospitalization, indicating that older age was associated with a lower incidence of hospitalization.16 Patients younger than 2 months had a longer length of hospital stay (6 versus 5 days, p<0.00001) and increased risk of ICU admission (OR 3.4; 95% CI: 2.5 to 4.6).29 In the evaluation of 229 patients admitted to the ICU for AVB, age was found to be inversely proportional to the time spent in the ICU and time in ventilatory support;30 the same was observed in a prospective study of 1,456 patients with AVB, which compared hospitalization in the ICU and in the hospital ward and showed that age younger than 2 months was as a risk factor for ICU admission (26% versus 53%; OR=4.1; 95% CI: 2.1 to 8.3).31 In the evaluation of 310 patients younger than 12 months with AVB, it was concluded that younger age was the main factor associated with the severity of clinical manifestations,32 which was also observed in the evaluation of 284 patients admitted to the ICU for AVB, where age less than 6 weeks was the main risk factor, accounting for 45% of the patients.23 The younger the child, the lower the levels of hemoglobin oxygen saturation in 206 patients hospitalized for AVB by RSV; it was shown that for each month younger the child was, there was a reduction in 0.41% in oxygen saturation.28 The relationship of age and presence of hospitalization for respiratory infection could be observed in the evaluation of 347 patients with acute respiratory infection, 234 inpatients and 112 outpatients, where the age of hospitalized patients was younger (8 versus 19 months, p<0.001).33
Maternal breastfeedingBreastfeeding is protective factor against severe AVB. A study of 12,474 children with bronchiolitis, of whom 1,588 required hospitalization, demonstrated that breastfeeding not starting in the maternity ward lead to a higher risk factor for hospitalization for AVB.20 A Brazilian study with 175 children hospitalized for AVB demonstrated that duration of exclusive breastfeeding was inversely related to the duration of oxygen use and of hospitalization, indicating that for every month of exclusive breastfeeding there was a decrease of 11hours in the time of oxygen use.34 Children weaned before one month of life had a 7.7-fold greater risk of being hospitalized for AVB.7 The importance of breastfeeding was further demonstrated in a study with 240 children hospitalized for AVB that concluded that breastfeeding an infant for less than four months was a risk factor for severe outcome and longer hospitalization time.26
Chronic pulmonary diseaseThe presence of chronic pulmonary disease, mainly bronchopulmonary dysplasia, is related to the greater severity of AVB.21,35 Infants with bronchopulmonary dysplasia have a 6.7-fold risk of death from AVB when compared to infants without this condition.36
Congenital heart diseaseThe presence of congenital heart disease is associated with increased severity of AVB.21 A recent study found that the length of hospitalization was higher in children who had congenital heart disease (6 versus 5 days; p<0.0001),29 and a retrospective study of 764 hospitalized patients with AVB showed that the presence of congenital heart disease was associated with a 50% longer length of hospital stay than in children without heart disease.37
GenderMale patients with AVB have a higher risk of requiring supplemental oxygen and mechanical ventilation when compared to female patients.19 A study of 12,474 infants with AVB, of whom 1,588 were hospitalized, demonstrated that the risk of hospitalization was higher in male patients.20
EthnicityThe role of ethnicity as a risk factor remains unclear, with controversial results in the literature. One study reported that patients of African descent have better progress when compared to Caucasians,28 while another study indicated the opposite.4 In some isolated populations, a few observations have been made: in New Zealand, patients of Maori origin had worse outcome;22 the same was observed for Native Americans and those originating in Alaska, who had more severe disease when compared to Caucasian patients.38
Etiology and presence of viral coinfectionThere are controversial results in the literature regarding the influence of the type of virus causing the disease on a more severe evolution.14 Some studies suggest that RSV is a factor of severity for AVB when compared to other viruses.11,32 A recent study demonstrated that severe AVB by RSV leads to prolonged hospital stay (6 versus 5 days, p<0.0001), higher risk of ICU admission (OR 2.7; 95% CI: 1.87 to 3.9), and increased need for oxygen therapy (OR 2.2; 95% CI: 1.8-2.6).29 A Brazilian study showed that pulse oximetry < 90% at hospital admission for lower respiratory tract infection was associated with RSV infection.39 However, other studies have shown that RSV does not lead to greater clinical severity when compared to other viruses, as in a Brazilian study of 89 hospitalized patients, which did not show any difference in severity between patients who had RSV and those who did not;40 and another study showed that positive RSV had no influence on the time of hospitalization.41 Regarding presence of viral coinfection, one study demonstrated that viral coinfection is responsible for increased severity of AVB.21 Other studies suggest that viral coinfection does not increase the severity of AVB,42 including a study in Brazil, which evaluated 176 patients and concluded that the clinical severity of AVB by RSV did not increase due to the presence of viral coinfection.43
Currently, through the development of quantitative PCR, the importance of the viral load on AVB severity has been studied, as well as the differentiation of coinfection and viral codetection, aspects that were little known and often disregarded in the literature.44
Other risk factors associated with AVB severity(i) low weight at admission;19 (ii) maternal smoking during pregnancy;20,45 (iii) atopic dermatitis;35 (iv) mechanical ventilation in the neonatal period;21 (v) maternal history of atopy;15 (vi) history of maternal asthma during pregnancy;45 (vii) season of birth;22 (viii) low socioeconomic status;7,20 (ix) Down syndrome;37,46 (x) environmental pollution;47 (xi) living at an altitude higher than 2,500 meters above sea level;48 and (xii) C-section delivery.49
Genetic factorsDespite the knowledge of the aforementioned risk factors, most infants hospitalized for AVB present no such factors. This led the researchers to believe that the epidemiological factors were not solely responsible for determining the clinical severity of AVB by RSV. Thus, genetic characteristics have been studied as an active risk factor in AVB severity. It has been estimated that there are over 10 million variations in the human genome (polymorphisms), and these may be associated with the clinical variability in diseases such as AVB, which have recently been the object of population studies. In this context, some studies addressing gene variations associated with immune response were performed in severe AVB by RSV.
A recent study evaluated 12,346 twins born in Denmark over a period of 10 years, and found an agreement between them regarding hospitalization for RSV. The agreement was 0.66 in homozygous twins and 0.53 in dizygotic ones, estimating a genetic contribution of 16% to 20% for disease severity,50 which contributes to the hypothesis of a genetic factor influencing AVB severity.
In AVB, infection is restricted to the surface cells of the respiratory epithelium, especially the hair cells of the bronchioles and type 1 pneumocytes in alveoli, and is opposed by the innate and adaptive immune response. RSV is recognized by epithelial cells through receptors specialized in recognition of pathogen-associated molecular patterns, known as pattern recognition receptors (PRRs) in the form of transmembrane molecules termed toll-like receptors (TLRs), present in macrophages and dendritic cells, which occur in the production of proinflammatory cytokines (Interleukin 6, 8, 10, and 13, tumor necrosis factor, RANTES, CX3CK1) and surfactant proteins.
Some of the factors have direct antiviral properties, while others stimulate the activation of natural killer cells, granulocytes, monocytes, and macrophages, initiating the adaptive immune response.
The main TLR responsible for the recognition of RSV is TLR4. TLR4 polymorphisms were associated with risk of severe AVB by RSV, but the results have been controversial. A better understanding of this question is important to more effectively identify those infants at risk for more severe AVB evolution. The presence of TLR4, Asp299Gly (rs4986790), and Thr399Ile (rs4986791) polymorphisms was verified in 99 infants hospitalized for severe AVB by RSV, 82 outpatients treated for this disease, and 90 healthy adults. TLR4 polymorphisms were more frequent in the group with severe AVB compared to the other two groups, leading the authors to conclude that the presence of TLR4 polymorphisms was associated with higher disease severity.51 Another study evaluated the influence of Asp299Gly and Thr399Ile polymorphisms in TLR4 gene on cytokine production and demonstrated that there was no influence, concluding that the determination of TLR4 gene polymorphisms does not have any benefit in clinical practice.52 Another study with 312 patients and 356 controls evaluated the influence of Asp299Gly polymorphism in TLR4 gene regarding the susceptibility to severe AVB by RSV and demonstrated that this polymorphism does not influence disease severity. The authors concluded that the severity of AVB by RSV appears to be associated with constitutional and environmental factors.53
One study analyzed 52 children with AVB by RSV (26 outpatients, 21 hospitalized in the hospital ward, and five admitted to the ICU), and found that the presence of Asp299Gly and Thr399Ile polymorphisms in the TLR4 gene, and a dysfunction of peripheral blood mononuclear cells stimulated by phytohemagglutinins expressed by hyper-responsiveness in response to lipopolysaccharides, were associated with increased severity of AVB by RSV.54
At the evaluation of D259G and T359I polymorphisms of TLR4 in 131 infants with severe AVB by RSV compared with 270 controls, polymorphisms alone were not associated with disease severity, but the presence of a gene haplotype with both polymorphisms showed a significant association with severity (p<0.001).55
One study investigated whether polymorphisms in other TLRs, in addition to TLR4, could be related to susceptibility to AVB by RSV. Thus, 19 polymorphisms in TLRs 1, 2, 3, 5, 6, 9, and 10 were evaluated; an association was found between polymorphisms in receptors 9 and 10.56
In the evaluation of 106 children hospitalized for AVB by RSV and 120 controls (healthy adults with no history of severe respiratory infection) to verify the association of three polymorphisms (-28C/G, -403G/A, and In1.1T/C) in the RANTES gene with disease severity, there was no association between disease severity and polymorphisms in isolation, but the combination of the three polymorphisms, forming a gene haplotype, was more common in cases than in controls, leading the authors to conclude that there was an association between polymorphisms in the RANTES gene and severity of AVB by RSV.57
One study found that Thr1Meth polymorphism (rs10735810) in the vitamin D receptor was associated with AVB, with the T “minor” allele showing a greater propensity to AVB.58
Another study analyzed 384 single nucleotide polymorphisms in 470 children hospitalized for AVB by RSV and 1,008 controls, and found that polymorphisms in the vitamin D receptor (rs10735810), JUN (rs11688), IFNA5 (rs10757212), and NOS-2 (rs1060826) genes showed association with AVB. The authors concluded that polymorphisms in innate immune response genes are important in determining susceptibility to the AVB.59
Researchers evaluated whether the presence of polymorphisms in the CX3CR1 receptor present in leukocytes, which binds to G protein of RSV, could have a correlation with AVB severity. They evaluated 82 children hospitalized for AVB by RSV, comparing them with 120 adults with no history of severe respiratory infection, and concluded that the T280M polymorphism was more frequent in cases than in controls (37.8% vs. 20.8%; OR: 2.03; 95% CI: 1.1 to 3.9) demonstrating an association between the polymorphism and severity of infection.60
Pulmonary surfactant proteins SP-A, B, C, and D, in addition to maintaining the alveolar surface tension, also participate in immune mechanisms regulating the release of proinflammatory cytokines and participate in chemotaxis and tissue repair. A recent study investigated whether polymorphisms in SP-A1, SP-A2, and SP-D genes were associated with the severity of RSV infection. A prospective study with 118 children younger than 6 months with RSV infection and 104 controls with no history of severe respiratory infection was conducted, and significant differences were found for some polymorphisms. When analyzed for the presence of gene haplotypes, there was a significant association between their occurrence and severity of RSV infection evolution (p<0.001).61
Interleukins (IL) have an important role in immune response, and associations between different polymorphisms and genic haplotypes of IL-4, IL-8, IL-10, IL-13, and IL-18 genes with increased severity of RSV infection have been found. The studies are controversial; the association usually has low OR and some studies have shown no association. In one study, the association was only significant in patients older than 6 months for IL-4 or younger than 6 months for IL-10, suggesting an age-dependent effect on Th1/Th2 balance.62
As polymorphisms in genes related to the presence and increased severity of AVB are associated with immune response, their identification will allow for a better understanding of which pathways are involved in the immune response to the virus and thus collaborate with a more complete understanding of the disease. Knowledge of polymorphisms associated more severe evolution illuminates the possibility of introducing new therapies conditioned by pharmacogenetics.
ConclusionSevere AVB is the most frequent complication of RSV infection, accounting for a large number of hospitalizations and high costs, and can lead to death.
Advances have occurred in recent years regarding the understanding of of the severity variability of AVB by RSV. The main epidemiological factors include prematurity, passive smoking, young age, lack of breastfeeding, chronic lung disease, and congenital heart disease. Other factors, such as gender and ethnicity, virus type, and presence of viral coinfection, remain controversial.
There are reports of other factors that can also influence severity, but these require further studies. They include low weight at admission, maternal smoking during pregnancy, atopic dermatitis, mechanical ventilation in the neonatal period, maternal history of atopy, maternal history of asthma during pregnancy, birth season, low socioeconomic status, Down syndrome, environmental pollution, living at an altitude higher than 2,500 meters above sea level, and cesarian section delivery. Regarding genetic factors, some polymorphisms appear to be associated with more severe evolution; the most often studied gene polymorphisms are TLR4, TLR9, TLR10, RANTES, VDR, JUN, IFNA5, NOS-2, and CX3CR1, as well as genes encoding surfactant proteins and IL-4, IL-8, IL-10, IL-13, and IL-18.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank the research group in chronic obstructive pulmonary diseases of Faculdade de Ciências Médicas da Unicamp, the pulmonary function lab of Centro de Investigação em Pediatria, and the multiuser laboratory of the Department of Medical Genetics - http://laboratoriomultiusuario.com.br.
Please cite this article as: Alvarez AE, Marson FA, Bertuzzo CS, Arns CW, Ribeiro JD. Epidemiological and genetic characteristics associated with the severity of acute viral bronchiolitis by respiratory syncytial virus. J Pediatr (Rio J). 2013;89:531–543.