This study aimed to determine the epidemiology and clinical characteristics of EV-D68-associated respiratory diseases among Asian children.
SourcesThe PubMed, Embase, Web of Science, Scopus, China National Knowledge Infrastructure (CNKI), VIP, WanFang, and SinoMed electronic databases were searched from inception to February 28, 2025, to identify relevant articles. Studies reporting the detection rate of enterovirus D68 (EV-D68) in pediatric patients with respiratory tract infections were included. The methodological quality regarding the risk of bias was assessed according to the approach proposed by the Joanna Briggs Institute.
Summary of the findingsTwenty studies involving 47,451 participants were included; additionally, 450 participants were positive for EV-D68 infection. The detection rate of EV-D68 ranged from 0.23 % to 10.56 %, with a pooled prevalence of 1.29 % (95 % CI 0.88–1.78 %), but significant heterogeneity (I² = 94.20 %, p < 0.001) indicated that this estimate was context-dependent. Subgroup analyses revealed that detection rates varied substantially by country, study period, and income level. Pneumonia is the most common respiratory disease presentation. EV-D68 is most prevalent during the summer and autumn.
ConclusionThis is the first review to focus specifically on Asian children, which suggests that EV-D68 detection rates vary significantly across Asian populations and are especially influenced by geographic location and surveillance timing. EV-D68 infection in children is most frequently associated with pneumonia. These findings support targeted regional surveillance networks during the summer-autumn seasons to improve clinical diagnostics and public health responses.
Trial RegistrationPROSPERO Identifier: CRD42023449889.
Enterovirus D68 (EV-D68), a member of the Picornaviridae family, has reemerged as a significant pediatric respiratory pathogen. Unlike most enteroviruses, EV-D68 is isolated from respiratory samples (specifically from the nose), and its biological and molecular characteristics are more similar to those of rhinoviruses. EV-D68 was initially identified in 1962 and gained global attention during the 2014 North American outbreak, which linked it to severe respiratory illness and acute flaccid myelitis (AFM) [1]. In recent years, the volume of epidemiological data related to EV-D68 has gradually increased [2–5]. The detection rate of EV-D68 varies widely throughout the world [6]. While western epidemiology is well characterized, Asian data remain fragmented, obscuring region-specific rates, clinical profiles, and seasonal trends. EV-D68 is most prevalent in children. Children aged < 16 years account for > 90 % of all EV-D68 infections worldwide [7]. Thus, the authors performed a systematic review and meta-analysis of the published literature to summarize the current knowledge of EV-D68-associated respiratory diseases in Asian children with respect to the detection rate, clinical characteristics, seasonality, and rate of coinfections.
MethodsSearch strategyThe authors conducted a systematic review and meta-analyses following the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) 2020 guidelines [8]. The review protocol was registered in PROSPERO (CRD42023449889). This meta-analysis was a secondary study based on previously published data; therefore, this study received an exemption from the Ethics Committee of the Third Affiliated Hospital of Zhengzhou University.
The authors searched the PubMed, Embase, Web of Science, Scopus, China National Knowledge Infrastructure (CNKI), VIP, WanFang, and SinoMed electronic databases for studies published up to February 28, 2025, with no language restrictions (Supplementary Table 1).
Inclusion and exclusion criteriaStudies were included if they met the following criteria: (1) they reported the detection rate of EV-D68 in pediatric patients (aged < 18 years) with respiratory tract infections across Asian populations; (2) they confirmed EV-D68 infection via reverse transcription–polymerase chain reaction (RT-PCR) in respiratory specimens; (3) they had sufficient detailed and extractable data to characterize the epidemiological patterns and clinical manifestations associated with EV-D68 infections; and (4) they included studies conducted over at least a full calendar year. Reviews, case reports, conference papers, notes, editorials, and nonobservational research were excluded. Studies in which EV-D68 was detected by the following methods (including viral culture, antigen detection, or antibodies) were excluded.
Literature screening and data extractionThe authors used EndNote to manage the search output. Two reviewers (L.X. and W.L.) independently screened the titles and abstracts of the studies and assessed their eligibility. After screening the published articles for eligibility, relevant data and information, including the first author’s name, year of publication, study design, sampling method, time of sample collection, country, study period, age group, EV-D68 diagnostic method, sample type, sample size, number of EV-D68-positive cases, basic information, demographic information, and clinical symptoms, were extracted from each eligible study and curated in a WPS Excel sheet. For studies that included both adults and children, only data related to children were extracted. Two authors (L.X. and W.L.) independently extracted and evaluated the data from the eligible studies. Any inconsistencies or disagreements were discussed with a third author (L.B.) and resolved via consensus.
Quality assessmentThe Joanna Briggs Institute quality assessment tool was employed to evaluate the quality of the studies that were included in the final analysis [9]. The scoring of individual studies was conducted according to frequency scales, with scores based on responses of yes, no, unclear, or not applicable. To calculate the total quality score for each study, the authors utilized the total number of positive scores.
Statistical analysisFor binary outcomes, proportional meta-analyses with 95 % confidence intervals were conducted via Stata 12.0 (StataCorp LP). The "metaprop" package was employed to implement double arcsine transformations on raw proportions prior to pooling, ensuring that normality assumptions were satisfied. Between-study heterogeneity was quantified through Cochran's Q test (χ²) and Higgins's I² metrics, with I² thresholds interpreted as follows: ≤ 25 % (negligible), 26–50 % (moderate), and > 50 % (substantial). Given the anticipated variations in clinical populations and research methodologies across studies, all analyses adopted DerSimonian–Laird random effects models to account for both within-study and between-study variance components. The authors further performed subgroup-level meta-analysis to address heterogeneity. The subgroups were based on the timing of sample collection, country, country income level, sex, age group, period of study (before and after 2014), and EV-D68 diagnostic method. Begg’s test and funnel plots were used to assess publication bias, with a p value < 0.05 and skewness of the funnel diagram indicating the presence of publication bias.
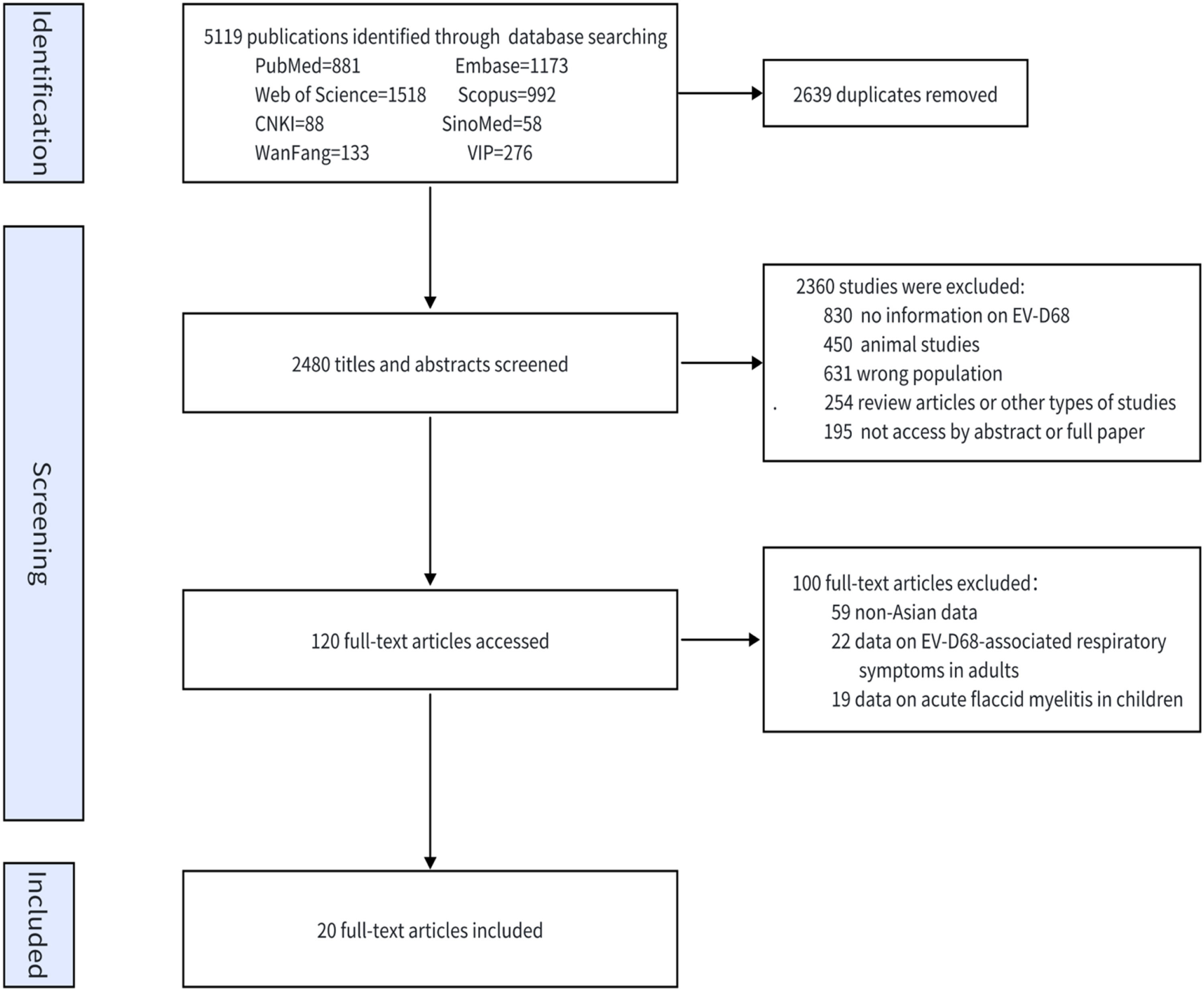
ResultsStudy search resultsIn total, 5119 studies were screened for eligibility. Of these, 120 full texts were reviewed, and 20 studies were ultimately included for analysis (Fig. 1) [10–29]. All the studies were written in English. Among the 20 included studies (Supplementary Table 2), 12 were retrospective. Eight studies were conducted in China, 5 were conducted in Japan, 3 were conducted in the Philippines, 2 were conducted in Thailand, 1 was conducted in Iran, and 1 was conducted in Myanmar. Five studies were performed in high-income countries, 11 were performed in upper-middle-income countries, and 4 were performed in lower-middle-income countries. The predominant technique used in these studies was RT-PCR. Four studies exclusively enrolled children younger than five years old.
A total of 47,451 participants were included in these 20 articles. Among 8768 children from 7 studies reporting age data, the overall median age was 0.92 years (IQR: 0.75–1.40). Gender data were reported in seven articles, with a male-to-female ratio of 1.50:1 (95 % CI 55.7–64.1 %) (Supplementary Table 2).
Quality of the included studiesBased on the Joanna Briggs Institute quality evaluation checklist, the articles that were included in the final analysis had a mean quality score of 7.85, with scores ranging from six to nine. One study was a high-quality study, and the remaining studies were moderate-quality studies (Supplementary Table 3).
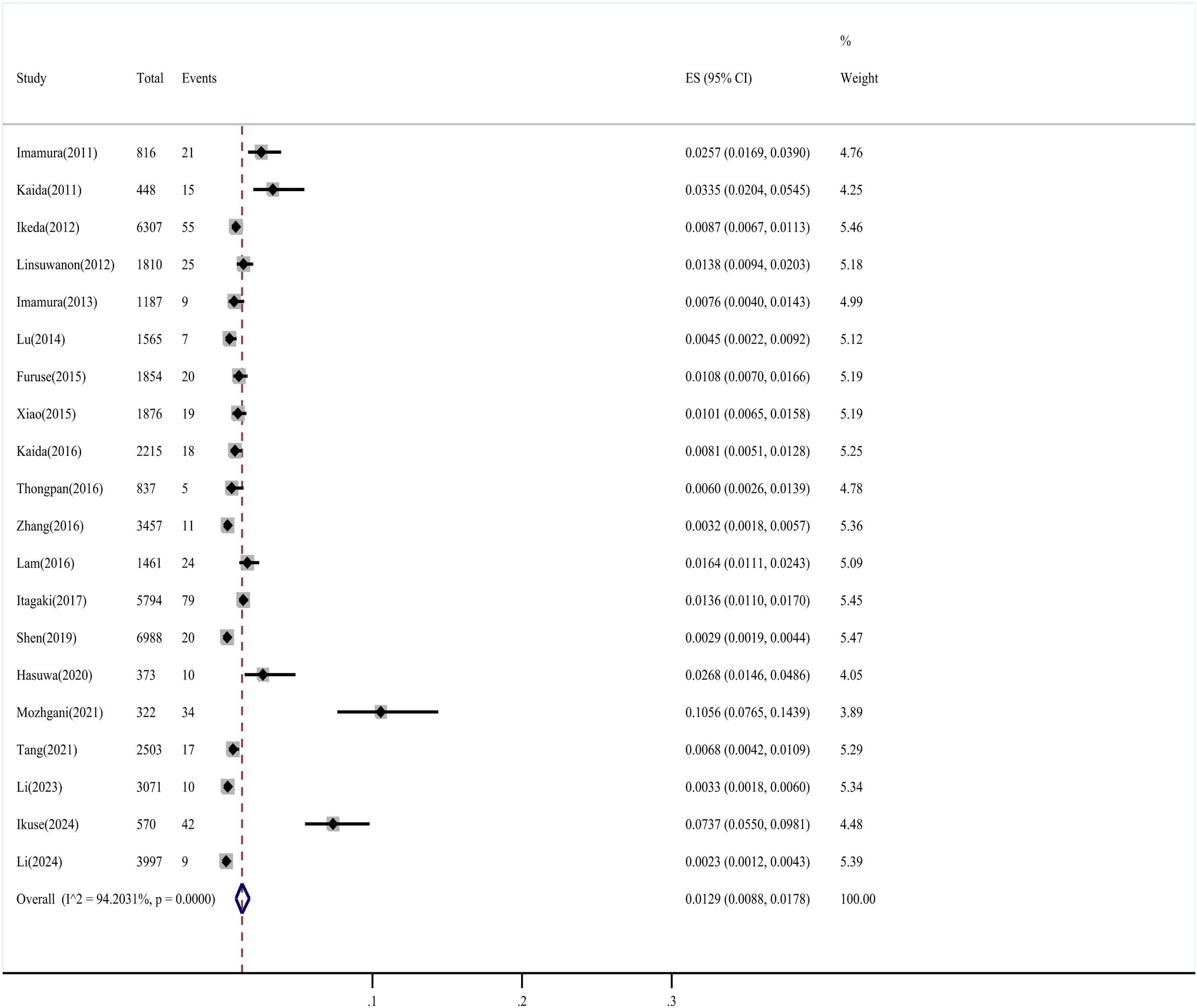
Detection rates and subgroup analysisA total of 450 EV-D68 infections were reported among 47,451 participants. The detection rate of EV-D68 ranged from 0.23 % to 10.56 % in the included studies, with a pooled prevalence (random-effects model) of 1.29 % (95 % CI 0.88–1.78 %), but significant heterogeneity (I² = 94.20 %, P < 0.001) indicated that this estimate was context-dependent (Fig. 2).
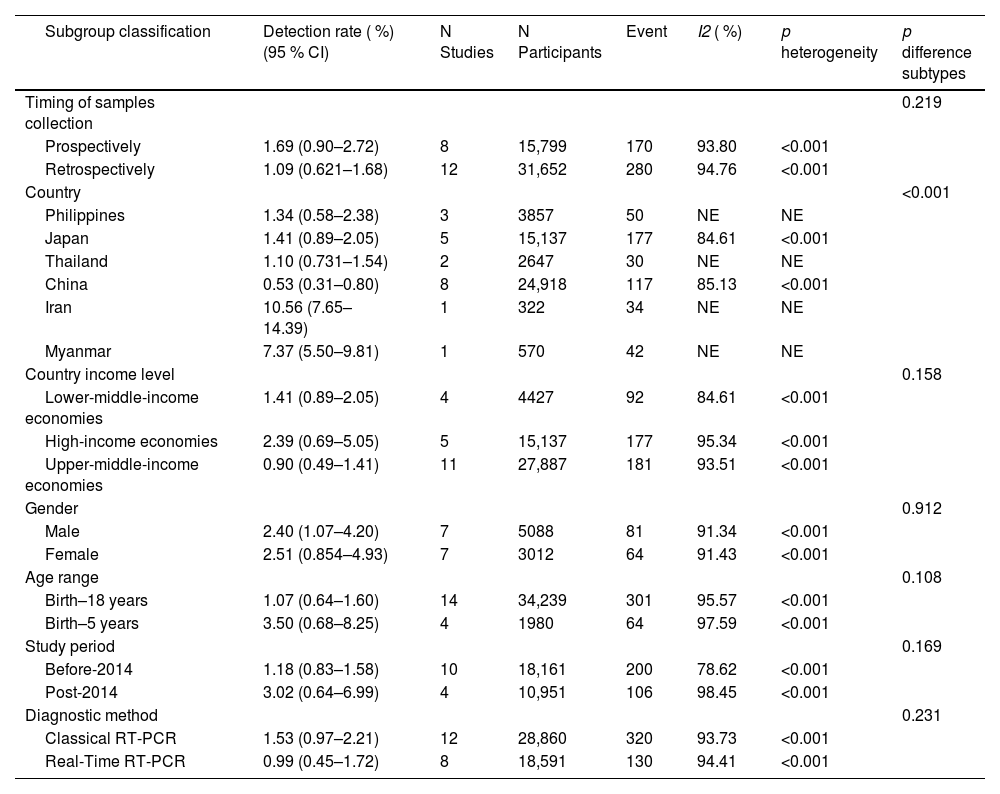
Subgroup analyses (Table 1) revealed that detection rates varied substantially by country (highest in Iran [10.56 %] and Myanmar [7.37 %]), study period (3.02 % post-2014 vs. 1.18 % pre-2014), and income level (2.39 % in high-income countries). The high detection in Iran and Myanmar derives from single-study estimates requiring cautious interpretation.
Subgroup analyses of factors associated with variability in EV-D68 detection among Asian children.
In the subgroup analysis, there were no heterogeneous results in three or fewer articles. NE = not estimable.
The detection rate of EV-D68 was greater in prospective studies and studies that included participants aged < 5 years, although the difference was not statistically significant. In the subgroup analysis based on sex, there was no significant difference in the detection rate between the male group and the female group (p = 0.912).
Year-to-year circulation patternsThe authors included 20 articles, and 17 articles that provided detailed annual detection rates were analyzed to derive annual detection rates to understand the EV-D68 cycle pattern from year to year. The annual detection rates of EV-D68 among Asian children with respiratory infections from 2007 to 2019 fluctuated significantly, with intermittent peaks observed in 2008 (2.57 %), 2010 (2.15 %), 2014–2015 (1.14 %–1.68 %), and notably 2018 (4.07 %). The highest rate occurred in 2018, suggesting that a major outbreak occurred in that year (Supplementary Fig. 1).
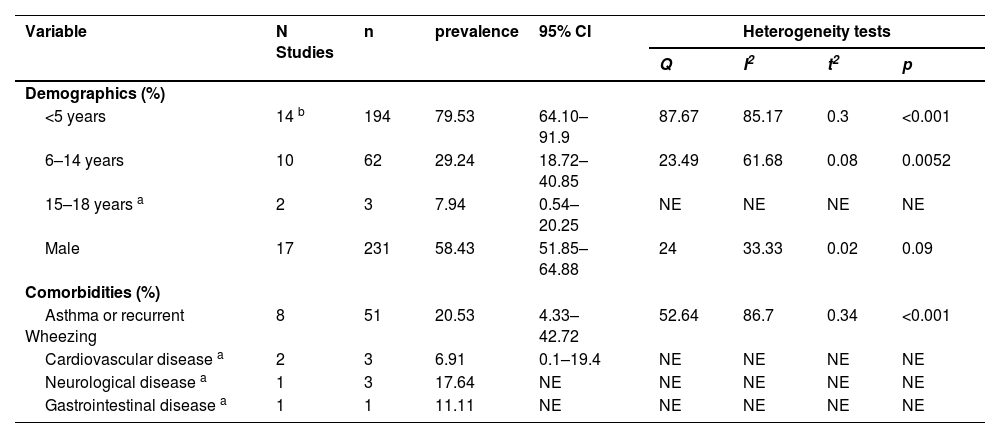
Demographic and clinical characteristics of EV-D68 patientsThe total number of EV-D68 infections in the eligible studies was 450. The ages ranged from 0 to 18 years. Among the 372 EV-D68-positive children from 17 studies reporting age data, the overall median age was 3.50 years (IQR: 2.67–5.20). A total of 79.53 % (95 % CI 64.10–91.9 %) of the children were < 5 years, 29.24 % (95 % CI 18.72–40.85 %) were 5–14 years old, and 7.94 % (95 % CI 0.54–20.25 %) were older than 14 years. The male-to-female ratio of EV-D68-infected patients was 1.41:1 (95 % CI 51.85–64.88 %). Nine studies reported the comorbidity of pediatric EV-D68 infection patients. A total of 60 of 194 patients (22.25 %, 95 % CI 7.85–40.4 %) had at least one comorbidity. Among them, the most common comorbidities were asthma or recurrent wheezing (20.53 %, 95 % CI 4.33–42.72 %) (Table 2, Supplementary Table 4).
Demographics and comorbidities of children with EV-D68 analyzed by meta-analysis.
| Variable | N Studies | n | prevalence | 95% CI | Heterogeneity tests | |||
|---|---|---|---|---|---|---|---|---|
| Q | I2 | t2 | p | |||||
| Demographics (%) | ||||||||
| <5 years | 14 b | 194 | 79.53 | 64.10–91.9 | 87.67 | 85.17 | 0.3 | <0.001 |
| 6–14 years | 10 | 62 | 29.24 | 18.72–40.85 | 23.49 | 61.68 | 0.08 | 0.0052 |
| 15–18 years a | 2 | 3 | 7.94 | 0.54–20.25 | NE | NE | NE | NE |
| Male | 17 | 231 | 58.43 | 51.85–64.88 | 24 | 33.33 | 0.02 | 0.09 |
| Comorbidities (%) | ||||||||
| Asthma or recurrent Wheezing | 8 | 51 | 20.53 | 4.33–42.72 | 52.64 | 86.7 | 0.34 | <0.001 |
| Cardiovascular disease a | 2 | 3 | 6.91 | 0.1–19.4 | NE | NE | NE | NE |
| Neurological disease a | 1 | 3 | 17.64 | NE | NE | NE | NE | NE |
| Gastrointestinal disease a | 1 | 1 | 11.11 | NE | NE | NE | NE | NE |
Q, Cochran’s Q statistic for heterogeneity; I2, I2 index for the degree of heterogeneity; t2, tau-squared measure of heterogeneity; NE, not estimable.
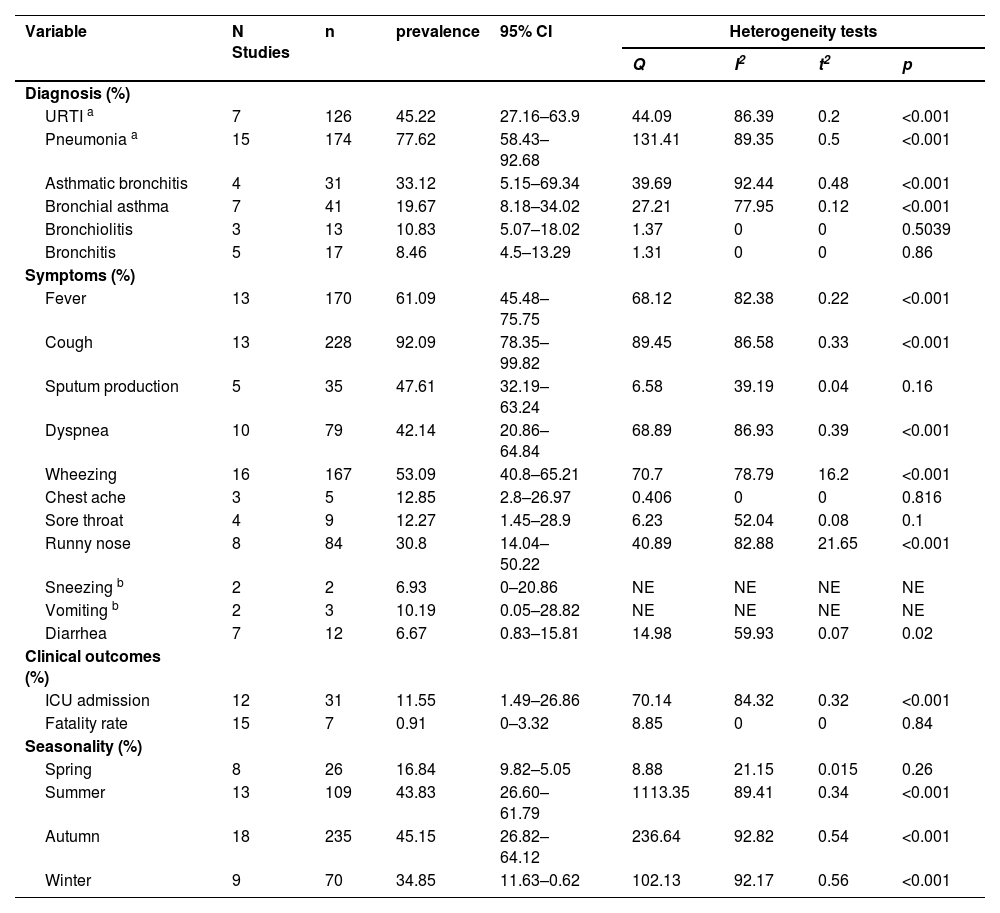
A total of 174 of 251 patients (77.62 %, 95 % CI 58.43–92.68 %) across 15 studies were diagnosed with pneumonia, which is the most common respiratory disease presentation. This was followed by upper respiratory tract infection (URTI), which was diagnosed in 126 of 235 patients (45.22 %, 95 % CI 27.16–63.90 %) across 7 studies. Other respiratory diseases included asthma, bronchitis (33.12 %), bronchial asthma (19.67 %), bronchiolitis (10.83 %), and bronchitis (8.46 %). The most prevalent clinical symptoms were cough (92.09 %, 95 % CI 78.35–99.82 %), fever (61.09 %, 95 % CI 45.48–75.75 %), wheezing (53.09 %, 95 % CI 40.80–65.21 %), sputum production (47.61 %, 95 % CI 32.19–63.24 %), dyspnea (42.14 %, 95 % CI 20.86–64.84 %), and runny nose (30.8 %, 95 % CI 14.04–50.22 %). The less frequent symptoms were chestache (12.85 %), sore throat (12.27 %), vomiting (10.19 %), sneezing (6.93 %), and diarrhea (6.67 %) (Table 3, Supplementary Table 5).
The clinical characteristics and seasonality of children with EV-D68 analyzed by meta-analysis.
| Variable | N Studies | n | prevalence | 95% CI | Heterogeneity tests | |||
|---|---|---|---|---|---|---|---|---|
| Q | I2 | t2 | p | |||||
| Diagnosis (%) | ||||||||
| URTI a | 7 | 126 | 45.22 | 27.16–63.9 | 44.09 | 86.39 | 0.2 | <0.001 |
| Pneumonia a | 15 | 174 | 77.62 | 58.43–92.68 | 131.41 | 89.35 | 0.5 | <0.001 |
| Asthmatic bronchitis | 4 | 31 | 33.12 | 5.15–69.34 | 39.69 | 92.44 | 0.48 | <0.001 |
| Bronchial asthma | 7 | 41 | 19.67 | 8.18–34.02 | 27.21 | 77.95 | 0.12 | <0.001 |
| Bronchiolitis | 3 | 13 | 10.83 | 5.07–18.02 | 1.37 | 0 | 0 | 0.5039 |
| Bronchitis | 5 | 17 | 8.46 | 4.5–13.29 | 1.31 | 0 | 0 | 0.86 |
| Symptoms (%) | ||||||||
| Fever | 13 | 170 | 61.09 | 45.48–75.75 | 68.12 | 82.38 | 0.22 | <0.001 |
| Cough | 13 | 228 | 92.09 | 78.35–99.82 | 89.45 | 86.58 | 0.33 | <0.001 |
| Sputum production | 5 | 35 | 47.61 | 32.19–63.24 | 6.58 | 39.19 | 0.04 | 0.16 |
| Dyspnea | 10 | 79 | 42.14 | 20.86–64.84 | 68.89 | 86.93 | 0.39 | <0.001 |
| Wheezing | 16 | 167 | 53.09 | 40.8–65.21 | 70.7 | 78.79 | 16.2 | <0.001 |
| Chest ache | 3 | 5 | 12.85 | 2.8–26.97 | 0.406 | 0 | 0 | 0.816 |
| Sore throat | 4 | 9 | 12.27 | 1.45–28.9 | 6.23 | 52.04 | 0.08 | 0.1 |
| Runny nose | 8 | 84 | 30.8 | 14.04–50.22 | 40.89 | 82.88 | 21.65 | <0.001 |
| Sneezing b | 2 | 2 | 6.93 | 0–20.86 | NE | NE | NE | NE |
| Vomiting b | 2 | 3 | 10.19 | 0.05–28.82 | NE | NE | NE | NE |
| Diarrhea | 7 | 12 | 6.67 | 0.83–15.81 | 14.98 | 59.93 | 0.07 | 0.02 |
| Clinical outcomes (%) | ||||||||
| ICU admission | 12 | 31 | 11.55 | 1.49–26.86 | 70.14 | 84.32 | 0.32 | <0.001 |
| Fatality rate | 15 | 7 | 0.91 | 0–3.32 | 8.85 | 0 | 0 | 0.84 |
| Seasonality (%) | ||||||||
| Spring | 8 | 26 | 16.84 | 9.82–5.05 | 8.88 | 21.15 | 0.015 | 0.26 |
| Summer | 13 | 109 | 43.83 | 26.60–61.79 | 1113.35 | 89.41 | 0.34 | <0.001 |
| Autumn | 18 | 235 | 45.15 | 26.82–64.12 | 236.64 | 92.82 | 0.54 | <0.001 |
| Winter | 9 | 70 | 34.85 | 11.63–0.62 | 102.13 | 92.17 | 0.56 | <0.001 |
Q, Cochran’s Q statistic for heterogeneity; I2, I2 index for the degree of heterogeneity; t2, tau-square measure of heterogeneity; NE, not estimable; URTI, upper respiratory tract infection.
A total of 31 of the 203 patients across the seven studies were transferred to the ICU (11.55 %, 95 % CI 1.49–26.86 %), and among the 248 patients, 7 died, for an overall fatality rate of 0.91 % (95 % CI 0–3.32 %) (Table 3, Supplementary Table 5).
Seasonality of EV-D68 infectionsAmong the 18 studies, 45.15 % (95 % CI 26.82–64.12 %) of the EV-D68 infections were detected in autumn, followed by summer (43.83 %, 95 % CI 26.60–61.79 %) across 13 studies, winter (34.85 %, 95 % CI 11.63–62.00 %) in 9 studies, and spring (16.84 %, 95 % CI 9.8–25.05 %) across 8 studies (Table 3, Supplementary Table 5).
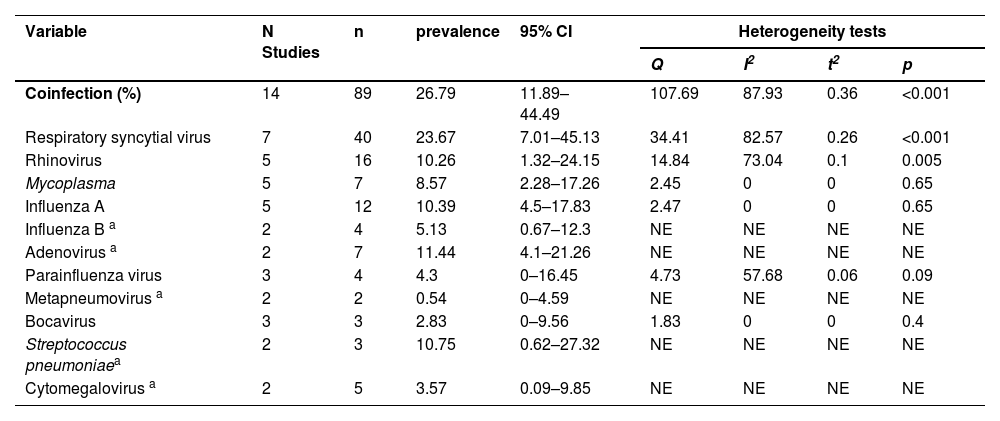
EV-D68 coinfectionThe occurrence of coinfection with EV-D68 and other pathogens was reported in 14 studies. The virus can establish coinfections with multiple viral, bacterial, and fungal pathogenic entities. The authors estimated that the rate of coinfections of EV-D68 with other pathogens was 26.79 % (95 % CI 11.89–44.49 %). The most commonly identified respiratory virus coinfected with EV-D68 was respiratory syncytial virus (23.67 %), followed by adenovirus (11.44 %) and influenza A virus (10.39 %) (Table 4, Supplementary Table 6).
Coinfections of children with EV-D68 analyzed by meta-analysis.
| Variable | N Studies | n | prevalence | 95% CI | Heterogeneity tests | |||
|---|---|---|---|---|---|---|---|---|
| Q | I2 | t2 | p | |||||
| Coinfection (%) | 14 | 89 | 26.79 | 11.89–44.49 | 107.69 | 87.93 | 0.36 | <0.001 |
| Respiratory syncytial virus | 7 | 40 | 23.67 | 7.01–45.13 | 34.41 | 82.57 | 0.26 | <0.001 |
| Rhinovirus | 5 | 16 | 10.26 | 1.32–24.15 | 14.84 | 73.04 | 0.1 | 0.005 |
| Mycoplasma | 5 | 7 | 8.57 | 2.28–17.26 | 2.45 | 0 | 0 | 0.65 |
| Influenza A | 5 | 12 | 10.39 | 4.5–17.83 | 2.47 | 0 | 0 | 0.65 |
| Influenza B a | 2 | 4 | 5.13 | 0.67–12.3 | NE | NE | NE | NE |
| Adenovirus a | 2 | 7 | 11.44 | 4.1–21.26 | NE | NE | NE | NE |
| Parainfluenza virus | 3 | 4 | 4.3 | 0–16.45 | 4.73 | 57.68 | 0.06 | 0.09 |
| Metapneumovirus a | 2 | 2 | 0.54 | 0–4.59 | NE | NE | NE | NE |
| Bocavirus | 3 | 3 | 2.83 | 0–9.56 | 1.83 | 0 | 0 | 0.4 |
| Streptococcus pneumoniaea | 2 | 3 | 10.75 | 0.62–27.32 | NE | NE | NE | NE |
| Cytomegalovirus a | 2 | 5 | 3.57 | 0.09–9.85 | NE | NE | NE | NE |
Q, Cochran’s Q statistic for heterogeneity; I2, I2 index for the degree of heterogeneity; t2, tau-squared measure of heterogeneity; NE, not estimable.
Publication bias was assessed using Begg's test, which indicated significant asymmetry (p = 0.002) (Supplementary Fig. 2). Visual inspection of the funnel plot corroborated this finding, suggesting potential publication bias or small-study effects (Supplementary Fig. 3). Egger’s linear regression test also indicated potential bias (p = 0.002), consistent with Begg’s test.
DiscussionA recent seroepidemiological systematic review on EV-D68 indicated that the seroprevalence increased quickly with age, reaching approximately 100 % by the age of 20 years, with no sign of decline throughout adulthood; this suggests continuous or frequent exposure of the populations to the virus [30]. However, the seroprevalence reflects only past infection; EV-D68 causes mild or asymptomatic infections, and cross-reactivity with antibodies against other viruses is possible. Through a systematic review and meta-analysis, the present study provides the first comprehensive evaluation of the epidemiological and clinical features of EV-D68 in respiratory specimens from Asian children.
The detection rate of EV-D68 ranged from 0.23 % to 10.56 % in the included studies, with a pooled prevalence (random-effects model) of 1.29 % (95 % CI 0.88–1.78 %). Significant heterogeneity was observed across studies (I² = 94.20 %), indicating that this estimate was a contextual summary. The meta-analysis confirms that EV-D68 detection in Asian children is highly heterogeneous. Many factors can influence the increase in detection rates. Previous studies have shown that the detection rate is related to children's age, national income level, geographical location, testing time, testing methods, and other factors [6]. The selection of specific subgroups was guided by factors that may significantly influence detection rates based on existing epidemiological knowledge of EV-D68. Subgroup analyses revealed national disparities, with Iran and Myanmar demonstrating markedly higher detection rates than China and other countries did (p < 0.001). However, the elevated rates in Iran and Myanmar derive from single-study estimates requiring cautious interpretation, potentially reflecting localized outbreaks or variations in assay sensitivity. Notably, studies conducted in high-income countries and after 2014 presented increased detection rates, which were correlated with enhanced molecular diagnostics and surveillance following North American outbreak awareness [31,32].
EV-D68 infections suggest circulation with a biennial epidemic cycle of EV-D68 infections in Europe and North America (2014, 2016, 2018), [33,34] less is known in Asia. The present study revealed irregular epidemic cycles of EV-D68 in Asia, potentially occurring every 2–4 years, which aligns with the global trends of biennial outbreaks reported in Europe and North America. The annual epidemic situation is partly affected by changes in the type of virus strains or the genetic variation of the enterovirus pathogen. However, caution is warranted in interpreting temporal trends given the sparse annual data and limited samples in the study, especially for zero-detection years.
The present analysis demonstrated increased EV-D68 susceptibility in children aged < 5 years. Consistent findings have been reported in another global review, which reported a higher detection rate of infections among children younger than 5 years of age than among those aged up to 18 years worldwide [6]. The vulnerability of children younger than 5 years of age to EV-D68 infections has been previously reported in several studies. Andres et al. [35]. reviewed EV-D68-associated respiratory cases at a hospital in Spain from 2014 to 2021 and reported that EV-D68 was mostly detected in pediatric populations (median age: 3 years), especially in patients aged < 5 years. A multicenter study of the European Nonpoliovirus Enterovirus Network (ENPEN) [36] reported that 58 institutes from 19 European countries reported 1004 EV-D68-positive samples, and that 78.9 % of infections were reported in children (ages 0–5 years). EV-D68 infections have mostly been reported in children, which is likely due to the immaturity of the immune system in children rather than in adults. The susceptibility of children to infection is also likely due to their lack of specific and cross-reactive immunity to enteroviruses [37–39]. However, the current literature demonstrates limited age-stratified reporting, with most studies predominantly reporting aggregate data — a methodological limitation potentially resulting in underestimation of the true disease burden in preschool-aged populations.
The authors found that the most common comorbidities were asthma or recurrent wheezing. However, wheezing was observed in 53 % (95 % CI 40.80–65.21 %) of the patients. Compared with other EV/RV patients, children with EV-D68 infections are more likely to have a history of asthma or recurrent wheezing [40]. A nationwide retrospective survey of hospitalizations for asthma among children was performed from January 2010 through October 2015 in Japan. The results revealed that the considerable increase in pediatric asthma hospitalizations in Japan in September 2015 was associated with the EV-D68 epidemic [41]. Using data from three surveillance systems in the United States, the analysis revealed an increase in medically attended acute respiratory illness and asthma/reactive airway disease exacerbations in children and adolescents during the summer of 2022. This increase may have been partially attributable to increased EV/RV circulation, specifically regarding the circulation of EV-D68 [42]. These results suggest that a history of recurrent wheezing or asthma is a risk factor for the detection of EV-D68 and that virus-induced wheezing/asthma might be a clinical feature of EV-D68 infection.
To explore the mechanism underlying the relationship between EV-D68 infection and asthma-like symptoms, Rajput et al. [43] developed a mouse model of EV-D68 infection and determined the mechanisms underlying airway disease. The results revealed that, in naive mice, neutrophilic inflammation and airway responsiveness were significantly greater after EV-D68 infection than after RV-A infection, which was dependent on IL-17. EV-D68 infection induces more IL-17-dependent airway inflammation and hyperresponsiveness, which is greater than that caused by RV-A infection, which is consistent with the clinical picture of severe asthma-like symptoms. Essaidi-Laziosi et al. [44] reported that global disruption of epithelial cell barrier function in patients with asthma is likely a key factor underlying increased permissiveness and susceptibility to EV-D68 infection. Currently, the specific pathogenic relationship between EV-D68 infection and asthma-like symptoms is still unclear and requires further investigation.
The present findings revealed pneumonia as the predominant manifestation of EV-D68 infection, indicating its predilection for lower respiratory tract infections (LRTIs) in pediatric populations. While 11.55 % (95 % CI 1.49–26.86 %) of cases required ICU admission, notable disparities emerged when global data were compared: a U.S. study reported substantially higher PICU admission rates (59.1 %), [45] whereas Finnish and Spanish cohorts demonstrated comparable rates (12.5 % [46] and 11.8 %, [35] respectively). This discrepancy may reflect heterogeneity in clinical cohorts and sample sizes across studies. Furthermore, the case fatality rate remained low at 0.91 %, which aligns with systematic review estimates (0 %–4.4 %), [6] suggesting a generally favorable prognosis for EV-D68-associated respiratory disease.
The present results suggest that EV-D68 is most prevalent during the summer and autumn. According to previous reports, infections caused by enteroviruses usually peak in late summer and can peak earlier in the United States in autumn [47,48]. Surveillance data from the United States revealed increases in severe respiratory illness and acute flaccid myelitis among children and adolescents with EV-D68 infections, which occurred biennially in the United States in 2014, 2016, and 2018 and primarily in the late summer and fall seasons [42]. These increases may be partially attributable to increased EV/RV circulation, specifically with respect to the circulation of EV-D68. These findings are consistent with the current findings. Pons-Salort et al. [49] used national enterovirus surveillance data from 1983 to 2013, as well as demographic and climatic data collected during the same period, to examine the patterns and drivers of the seasonality of these infections. Using mixed-effects models, they reported that climate (especially dew point temperature and latitude), but not demography, is likely to drive the seasonal pattern of enterovirus infections. This observation partly explains the seasonality of EV-D68.
In the present study, the overall coinfection rate of EV-D68 with other pathogens was 26.79 %. The most frequently detected respiratory viruses were respiratory syncytial virus and adenovirus. A multicenter study in Europe reported coinfections in 241/969 (24.9 %) EV-D68 patients, approximately half of whom also had human rhinovirus infections, followed by adenovirus and respiratory syncytial virus infections [36]. This rate is similar to the coinfection rate reported in the present study. Schuster et al. [40] reported 4 cases of coinfection with other pathogens among 339 cases of EV-D68 infection in children, and the combined infection rate was 1.2 %. The EV-D68 coinfection rates reported in different studies vary widely; however, the overall coinfection rate is not high, which is likely due to the late-summer, early-fall seasonality of the outbreaks. These findings highlight the pathogenicity of EV-D68.
There are currently no vaccines or antivirals against EV-D68 [50]. Therefore, non-pharmacological prevention strategies must be implemented. EV-D68 is usually isolated from respiratory secretions; therefore, it can spread through sneezing, coughing, and contact with surfaces contaminated by infected patients. Personal protective equipment (PPE) helps prevent the spread of EV-D68 infections. Moreover, handwashing, the use of alcoholic solutions, and wearing gloves are effective measures for preventing the spread of infection in healthcare settings [51–53].
This meta-analysis has certain limitations. First, more than half of the included studies were conducted in China or Japan. Therefore, the results of the analysis cannot accurately reflect the detection rate and clinical characteristics of EV-D68-associated respiratory infections in all Asian children. Second, more than half of the EV-D68 infections identified in the included studies were retrospectively reported; thus, the detection rate estimates were likely highly underestimated. Third, 4 of 20 studies exclusively enrolled children younger than five years old. Given that the reported median age of children infected by EV-D68 is young, the results may be skewed. Fourth, the meta-analysis demonstrated considerable heterogeneity, and the results of the subgroup analysis could not fully explain the source of the heterogeneity. In addition, the detection rate of EV-D68 via PCR is contingent upon the predominant EV-D68 clade in circulation. The currently circulating B3 clade, which is now spreading throughout the world, remains undetectable via previously established standard PCR assays because of sequence mismatches in primer/probe-binding regions. Surveillance estimates may be biased downward in years/regions where assays/primers were not updated (e.g., clade B3). Finally, the authors included data from before and after the COVID-19 pandemic. Public health measures implemented to limit the spread of SARS-CoV-2 have also disrupted the spread of respiratory viruses and enteroviruses. Additionally, the significant publication bias observed may be attributable to small-study effects, potentially leading to an overestimation of the pooled prevalence of EV-D68.
ConclusionsOverall, the current evidence suggests that the circulation of EV-D68 is sporadic but clinically significant. EV-D68 detection in Asian children varies markedly by region and timeframe. EV-D68 infection in children is most frequently associated with pneumonia, with a generally favorable overall prognosis. The authors recommend region-specific surveillance during peak periods to guide clinical preparedness and public health responses.
Funding sourcesThis study was supported by “Zhengzhou Medical and Health Science and Technology Innovation Guidance Program” (2024YLZDJH001).
Data availability statementThe data that support the findings of this study are available from the corresponding author.
The authors declare no conflicts of interest.